Alpha particles are relatively slow and heavy compared with other forms of nuclear radiation.
What are alpha particles?
Alpha particles (a) are composite particles consisting of two protons and two neutrons tightly bound together (Figure 1). They are emitted from the nucleus of some radionuclides during a form of radioactive decay, called alpha-decay. An alpha-particle is identical to the nucleus of a normal (atomic mass four) helium atom i.e. a doubly ionised helium atom.
Alpha particles (also termed alpha radiation or alpha rays) was the first nuclear radiation to be discovered, beta particles and gamma rays were identified soon after.

(Figure 1)
What are the properties of alpha particles?
Alpha particles are relatively slow and heavy compared with other forms of nuclear radiation. The particles travel at 5 to 7 % of the speed of light or 20,000,000 metres per second and has a mass approximately equivalent to 4 protons.
Alpha particles, because they are highly ionising, are unable to penetrate very far through matter and are brought to rest by a few centimetres of air or less than a tenth of a millimetre of biological tissue (Figure 2).
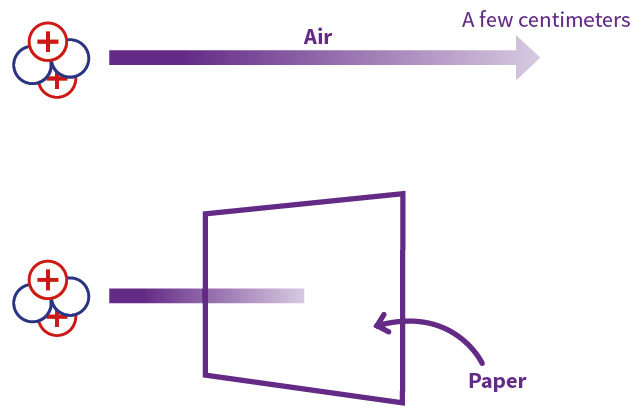
(Figure 2)
What are the health effects of exposure to alpha particles?
Alpha particles are highly ionising because of their double positive charge, large mass (compared to a beta particle) and because they are relatively slow. They can cause multiple ionisations within a very small distance. This gives them the potential to do much more biological damage for the same amount of deposited energy.
Alpha particles can't penetrate the normal layer of dead cells on the outside of our skin but can damage the cornea of the eye. Alpha-particle radiation is normally only a safety concern if the radioactive decay occurs from an atom that is already inside the body or a cell. Alpha-particle emitters are particularly dangerous if inhaled, ingested, or if they enter a wound.
What are some common sources of alpha particles?
Many alpha emitters occur naturally in the environment. For example, alpha particles are given off by radionuclides such as uranium-238, radium-226, and other members of the naturally occurring uranium, thorium and actinium decay series which are present in varying amounts in nearly all rocks, soils, and water.
Artificially produced sources of alpha particles include the radioisotopes of elements such as plutonium, americium, curium and californium. These are generally produced in a nuclear reactor through the absorption of neutrons by various uranium radioisotopes.
What are some uses of alpha particles?
Alpha particles have low penetrating power but this still provides a range of useful applications:
- smoke detectors – americium-241 is commonly used in ionising smoke detectors. Smoke that enters the detector reduces the amount of alpha particles that are detected and triggers the alarm
- static eliminators typically use alpha particles from polonium-210 to remove static charges from equipment
- radioisotope thermoelectric generators use alpha particle decay from plutonium-238 to generate heat which is converted to electricity, commonly used in space probes
- some alpha emitters are being investigated for their potential use in unsealed source radiotherapy to treat cancer.


