The key difference between gamma rays and X-rays is how they are produced.
What are gamma rays?
A gamma ray (g) is a packet of electromagnetic energy (photon) emitted by the nucleus of some radionuclides following radioactive decay. Gamma photons are the most energetic photons in the electromagnetic spectrum.
What are the properties of gamma rays?
Gamma rays are a form of electromagnetic radiation (EMR). They are the similar to X-rays, distinguished only by the fact that they are emitted from an excited nucleus. Electromagnetic radiation can be described in terms of a stream of photons, which are massless particles each travelling in a wave-like pattern and moving at the speed of light. Each photon contains a certain amount (or bundle) of energy, and all electromagnetic radiation consists of these photons. Gamma-ray photons have the highest energy in the EMR spectrum and their waves have the shortest wavelength.
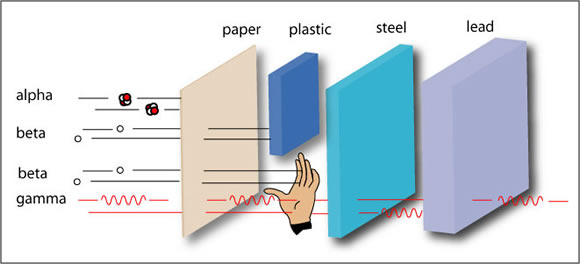
Scientists measure the energy of photons in electron volts (eV). X-ray photons have energies in the range 100 eV to 100,000 eV (or 100 keV). Gamma-ray photons generally have energies greater than 100 keV. For comparison, ultraviolet radiation has energy that falls in the range from a few electron volts to about 100 eV and does not have enough energy to be classified as ionising radiation. The high energy of gamma rays enables them to pass through many kinds of materials, including human tissue. Very dense materials, such as lead, are commonly used as shielding to slow or stop gamma rays.
What is the difference between gamma rays and X-rays?
The key difference between gamma rays and X-rays is how they are produced. Gamma rays originate from the settling process of an excited nucleus of a radionuclide after it undergoes radioactive decay whereas X-rays are produced when electrons strike a target or when electrons rearrange within an atom. Cosmic rays also include high-energy photons and these are also called gamma-rays whether or not they originated from nuclear decay or reaction.
What are the health effects of exposure to gamma radiation?
Gamma radiation is highly penetrating and interacts with matter through ionisation via three processes; photoelectric effect, Compton scattering or pair production. Due to their high penetration power, the impact of gamma radiation can occur throughout a body, they are however less ionising than alpha particles. Gamma radiation is considered an external hazard with regards to radiation protection.
Similar to all exposure to ionising radiation, high exposures can cause direct acute effects through immediate damage to cells. Low levels of exposure carry a stochastic health risk where the probability of cancer induction rises with increased exposure.
What are some common sources of gamma radiation?
Gamma radiation is released from many of the radioisotopes found in the natural radiation decay series of uranium and thorium as well as being emitted by the naturally occurring radioisotope potassium-40. These are found in all rocks and soil and even in our food and water.
Artificial sources of gamma radiation are produced in fission in nuclear reactors, high energy physics experiments, nuclear explosions and accidents.
What are some uses of gamma ray emitters?
Gamma emitting radionuclides are the most widely used radiation sources. The penetrating power of gamma rays has many applications. However, while gamma rays penetrate many materials, this does not make them radioactive. The four radionuclides that are by far the most useful are cobalt-60, caesium-137, technetium-99m and americium-241.
Uses of cobalt-60:
- sterilisation of medical equipment in hospitals
- pasteurisation, via irradiation, of certain foodstuffs
- levelling or thickness gauges (i.e. food packaging, steel mills)
- industrial radiography.
Uses of caesium-137:
- measurement and control of the flow of liquids in industrial processes
- investigation of subterranean strata (i.e. oil, coal, gas and other mineralisation)
- measurement of soil moisture-density at construction sites
- levelling gauges for packaging of food, drugs and other products.
Uses of technetium-99m:
- Tc-99m is the most widely used radioactive isotope for medical diagnostic studies
- different chemical forms are used for brain, bone, liver, spleen and kidney imaging. It is also used for blood flow studies.
Uses of americium-241:
- smoke detectors for households
- fluid levelling and density gauges
- thickness gauges for thin materials (i.e. paper, foil, glass)
- aircraft fuel gauges
- when mixed with beryllium, americium-241 produces a 241AmBe neutron source with uses in well logging, neutron radiography and tomography.


