Alpha, beta and gamma radiation are the most common types of radioactive decay but there are other ways that unstable atoms can become stable.
Spontaneous fission
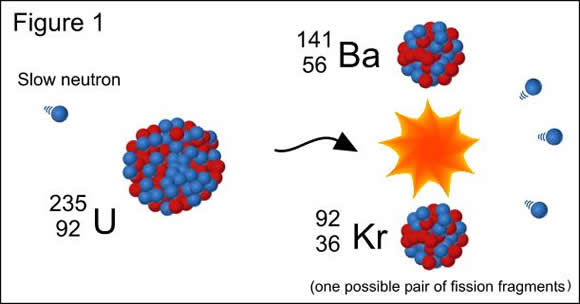
Spontaneous fission can occur only in very heavy elements with an atomic mass number greater than 92. It is different to the nuclear fission that occurs in a nuclear reactor which is induced by neutron bombardment of the fuel. Spontaneous fission occurs as a result of quantum tunnelling without the atom having to be struck by a neutron. The results of spontaneous fission are the same as that for induced fission, with the element splitting into two lighter nuclei and releasing neutrons in the process. These neutrons may induce a nuclear fission chain reaction if there is enough fissile material present.
Spontaneous fission does occur rarely in the naturally occurring radioactive decay series for thorium-232, uranium-235 and uranium-238. It is more likely to occur in artificially produced elements such as plutonium-240, curium-250 and californium-252.
Neutron emission
Neutron emission is a decay process where one or more neutrons are ejected from a nucleus. It can occur in nuclei that are neutron rich/proton poor. As only one or more neutrons are lost the atom does not transmute into a different element but becomes a different isotope of the original element.
Neutrons are a subatomic particle that are one of the basic building blocks of a nucleus. They are neutral, having no electrical charge and have a mass similar to the combined mass of one proton plus one electron.
An isolated neutron is unstable and decays by emitting an electron and becoming a proton with a half-life of 10.5 minutes. When this occurs while the neutron is part of an atom it is called beta decay.
Positron or beta plus (β+) emission
A positron is the anti-matter equivalent of an electron, having exactly the same mass as an electron but an opposite electrical charge. Positrons are emitted from some unstable isotopes that have too few neutrons to be stable. Positron emission is equivalent to the capture of an electron in electron capture. In both cases a proton is transformed into a neutron. A significant difference is that positron emission requires more energy than electron capture.
Positrons are sometimes called beta 'plus' particles to distinguish them from more common beta-'minus' particles (electrons).
Positrons can also be produced, along with a matching electron, when gamma rays of more than 1 mega-electron volt (MeV) interact with matter in a process called pair-production.
Radioisotopes that emit positrons are useful in a nuclear medicine imaging procedure called PET. A positron and electron will mutually annihilate each other if they come into close proximity with their mass disappearing and being converted into energy in the form of two gamma rays, emitted back-to-back, in opposite directions. These gamma rays are called annihilation radiation. In PET, the two annihilation gamma rays are detected simultaneously allowing the position of the annihilation to be determined.
Electron capture
Electron capture occurs when there are too many protons in the nucleus, and there isn't enough energy to emit a positron.
In this case, one of the orbital electrons is captured by a proton in the nucleus, this creates a neutron and a neutrino which is emitted. By changing the number of protons, electron capture transforms the nuclide into a new element.
Since one of the lower shell electrons is captured (either K or L shells) the replacement of the electron by an electron from a higher energy state releases a characteristics x-ray.
Internal conversion
This is a process where an excited nucleus interacts with an orbital electron and ejects the electron. The atom does not transmute into a new element and the ejected electron is not a beta particle as it did not come from the nucleus.
Since an electron is lost, a higher energy electron will move into the position of the previous electron and emit a characteristic x-ray or Auger electron.
Other types of decay
A number of other radioactive decay modes exist but are quite rare. These include proton emission, double proton emission, cluster decay, bound state beta decay, double beta decay, double electron capture, electron capture with positron emission, double positron emission and isomeric transition.


